Aluminum Metal Crystallizes in a Cubic Close-packed Structure
If Al atom has a radius 143 A what is the length of a side of the unit cell. I For cubic close-packed structure a2 2 r2 2 125354 pm Here a is the edge length of the unit cell and r is the atomic radius.

Aluminium Crystallizes In A Cubic Close Packed Structre Its Metallic Radius Is 125 Ppm A Wha Youtube
A 2 2 125.

. You can get ncert. 10 - The density of aluminum is 27 gcm3. Aluminum atomic radius 143 Å Å crystallizes in a cubic closely packed structure.
C assume that the aluminum atoms can be represented as spheres. 10 - Platinum atomic radius 138 crystallizes in a. A How many aluminum atoms are in.
Calculate the edge length of the face-centered cubic unit cell and the density of platinum 2. Check_circle Expert Solution Want to see the full answer. Cubic Closest Packed structure is equivalent to Face-Centered Cubic unit cell.
Sign up for free. 10 - Barium crystallizes in a body-centered cubic unit. 1 There are 4 atomic radii r along the face of an fcc cube.
Aluminum metal crystallizes in a cubic close-packed structure face-centered cubic cell see image below. Its metallic radius is 125 pm. Verified by Toppr It is given that aluminum crystallises in a cubic closed packed structure.
Assume that the aluminum atoms can be represented as spheres if each Al A. See solution arrow_back Chapter 10 Problem 83E Chapter 10 Problem 85E. 10 - The free space in a metal may be found by.
Calculate the edge length of the facecentered cubic unit cell and the density of aluminum. Nickel metal has a cubic close-packed structure with a facecentered. Classes Class 5 Class 6 Class 7 Class 8 Class 9 Class 10 Class 11 Commerce Class 11 Engineering Class 11 Medical Class 12 Commerce Class 12 Engineering.
Its metallic radius is 125 pm. The closest packing of most metallic cystals helps to. Its metallic radius is 125 pm.
B what is the coordination number of each aluminum atom. 123 Then we have our molecule of interest here. Equal numbers of metal crystallize in each of the three structure hexagonal closest packed face-centered cubic and body-centerd cubic.
Check out a sample textbook solution. Aluminum crystallizes in a face-centered cubic unit cell and has an atomic radius of 143 pm. Its metallic radius is 125 pm.
Aluminum metal crystallizes in a cubic close packed structure face centred cubic cell A how many Al atoms are in a unit cell. 10 - Cadmium sulfide sometimes used as a yellow. Sort of for example in a cubic close parked where we have ABC packing.
Aluminium crystallises in a cubic closepacked structure. Were continuing on with a lot of structures and a crystalline solid here on DSO The number of other particles that a particle of interest can touch is known as the coordination number. 99 279 ratings Sign up for free to view this solution.
Platinum atomic radius 138 Å crystallizes in a cubic closely packed structure. How many unit cells are there in 100 cm3 of Al. Figure 48 Schematic representation of the two layers closest-packed arrangements Table 42 Crystal Structure of Metals The crystal structure of metals are summarized in Table 42.
Answer In a cubic close packed structurethe length of the side of unit cell is related to radius by an equation r a22 Or a r x 22. Aluminum metal packs in a cubic closest-packed structure in which one plane of atoms can slip past another. Aluminum metal crystallizes in a cubic close-packed structure face-centered cubic cell Figure 1214mathrma mathrma How many aluminum atoms are in a unit cell.
So we have a baby. B How many unit cells are there in. Its metallic radius is 125 pm.
Therefore the edge length of face centered cubic structure of aluminium is 3535 pm. E 2 e 2 4r 2. The edge length of t Subjects English History Mathematics Biology Spanish Chemistry Business Arts Social Studies Physics Geography Computers and Technology Health Advanced Placement AP World Languages SAT German.
I What is the length of the side of the unit cell. Correct answer - Aluminum atomic radius 143 Å crystallizes in a cubic closely packed structure. MathrmAl is used in the turbines of aircraft engines because of its strength and low density.
A What is the length of the side of the unit cell. Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 125 pm.
Aluminum atomic radius 143 Å crystallizes in a cubic. Aluminium crystallizes in a cubic close-packed structure with a unit cell edge length of 3536 pm. Aluminium crystallises in a cubic close- packed structure.
A 2 1414 125. This is as follows. Thus the edge e can be found.
What is the density of aluminum. The edge length in pm of the unit cell and number of unit cells per cc of aluminium respectively are. The relation between edge length of crystal structure and radius of sphere is different in different structures.
Precipitation hardening produces alloys that are five to six times as strong as aluminum and make an excellent structural metal. Ii Volume of one unit cell a 3354 pm 34410 23 cm 3 1 pm10 10 cm. 10 - Aluminum atomic radius 143 crystallizes in a.
Aluminum metal crystallizes in a cubic close-packed structure face-centered cubic cell. What is the radius of Al atom. Ii How many unit cells are there in 100 cm3of aluminium.
Aluminium crystallizes in a cubic close-packed structure. As a result pure aluminum metal is too weak to be used as a structural metal in cars or airplanes. I What is the length of the side of the unit cell ii How many unit cells are there in 100 cm3 of aluminum is solved by our expert teachers.
Aluminium crystallizes in a cubic close packed structure. Nickel metal crystallizes in a cubic closest packed structure.

Aluminium Crystallizes In A Cubic Close Packed Structre Its Metallic Radius Is 125 Ppm A Wha Youtube

10 6 Lattice Structures In Crystalline Solids General College Chemistry I

Aluminium Crystallizes In A Cubic Close Packed Structure Its Metallic Radius Is 125pm Youtube
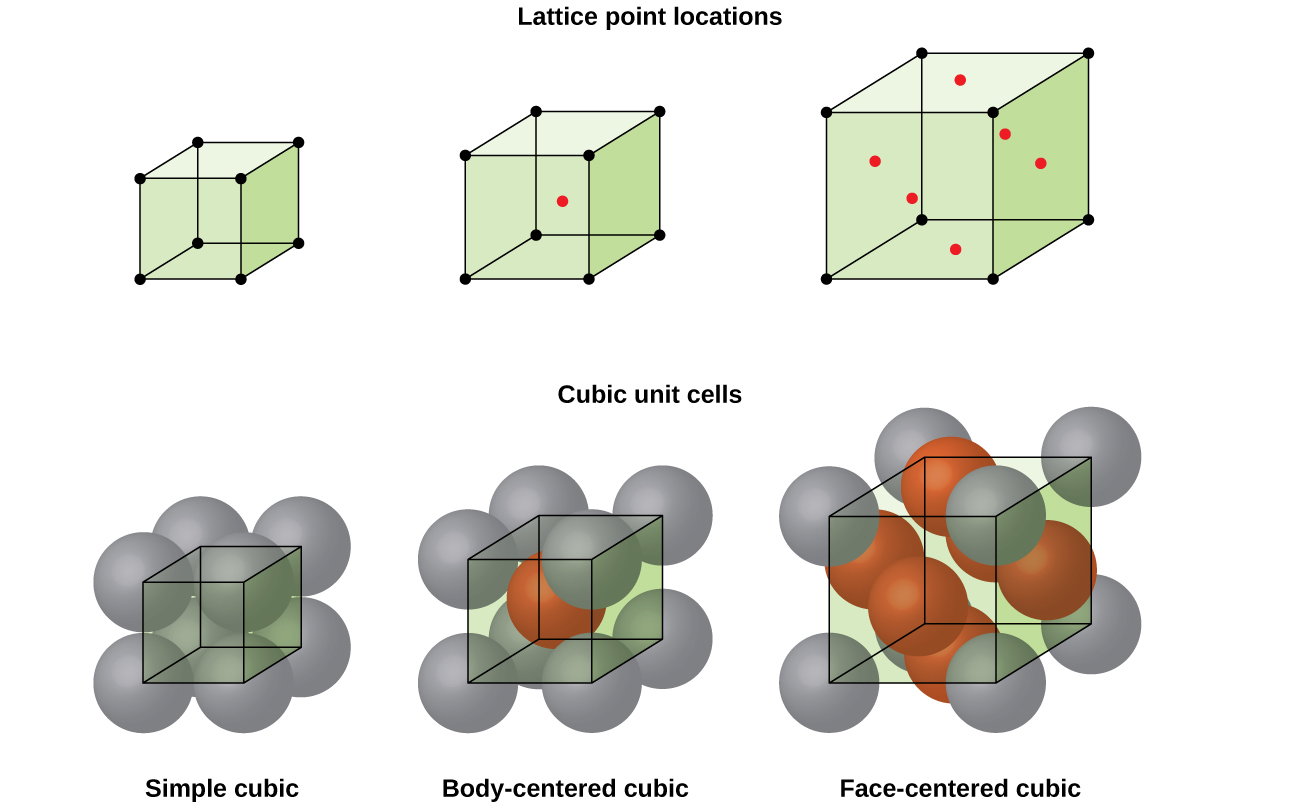
4 1 Unit Cells Chemistry Libretexts
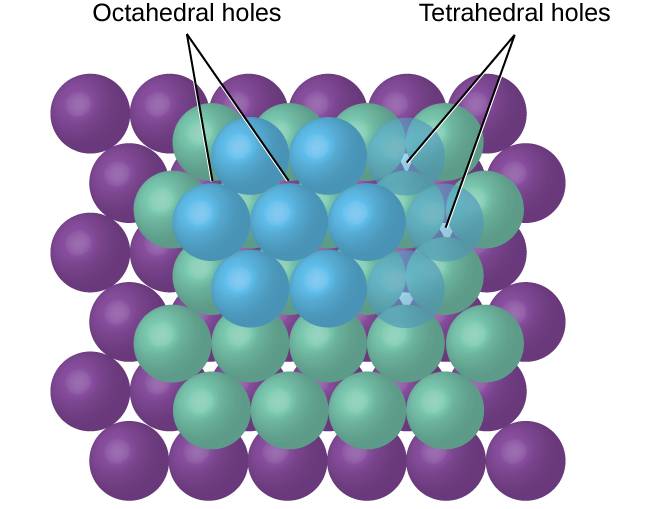
10 6 Lattice Structures In Crystalline Solids Chemistry

Aluminium Crystallizes In A Cubic Close Packed Structure Its Metallic Radius Is 125 Pm A What Is The Length Of The Side Of The Unit Cell B How Many Unit Cells Are There In 1 00

Aluminium Crystallizes In A Cubic Close Packed Structure Its Metallic Radius Is 125 Pm The Edge Length In Pm Of The Unit Cell And Number Of Unit Cells Per Cc Of Aluminium

Aluminum Metal Crystallizes In A Cubic Close Packed Structure Face Centered Cubic Cell Assume That The Aluminum Atoms Can Be Represented As Spheres If Each Al Atom Has A Radius Of 1 43 A A
Aluminium Crystallizes In A Cubic Close Packed Structure Its Metallic Radius Is 125 Pm Sarthaks Econnect Largest Online Education Community

Aluminum Metal Crystallizes In A Cubic Close Packed Structure Face Centered Cubic Cell See Image Below Image Src Crystalization1102377373104052220 Jpg Alt Crystalization Caption A H Study Com

Solved Aluminum Metal Crystallizes In A Cubic Close Packed Chegg Com
Aluminium Crystallizes In A Cubic Close Packed Structure Its Metallic Radius Is 125pm Calculate The Edge Length Of Unit Cell Sarthaks Econnect Largest Online Education Community
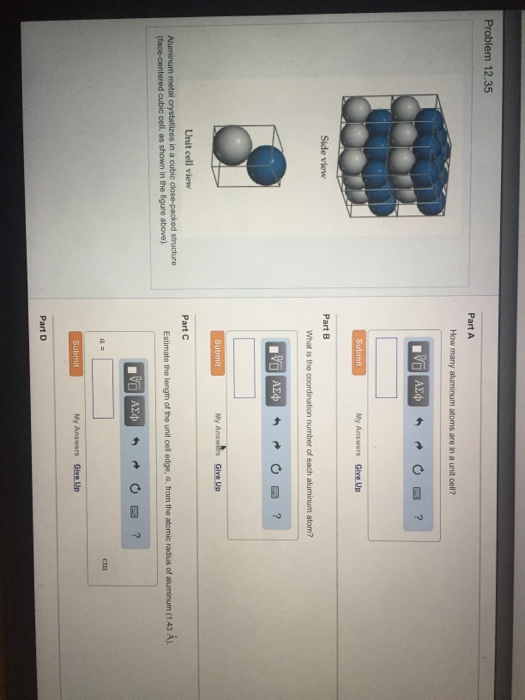
Solved Aluminum Metal Crystallizes In A Cubic Close Packed Chegg Com

10 6 Lattice Structures In Crystalline Solids General College Chemistry I

If The Radius Of A Metal Is 2 00a And Its Crystal Structure Is In Cubic Close Packed Fc Lattice What Is The Volume In Cm 3 Of One Unit Cell

The Unit Cell Of Aluminium Is A Cube With An Edge Length Of 405 Pm The Density Of Aluminium Is Youtube

33 Aluminium Crystallises In A Cubic Close Packed Structure Radius Of Atom In The Metal Is 125 Pm Chemistry The Solid State 12310161 Meritnation Com

Aluminium Crystallises In A Cubic Close Packed Structure Its Metallic Radius Is 125 Pm I What Is Brainly In
Comments
Post a Comment